Press release
Related Press

22 February 2024
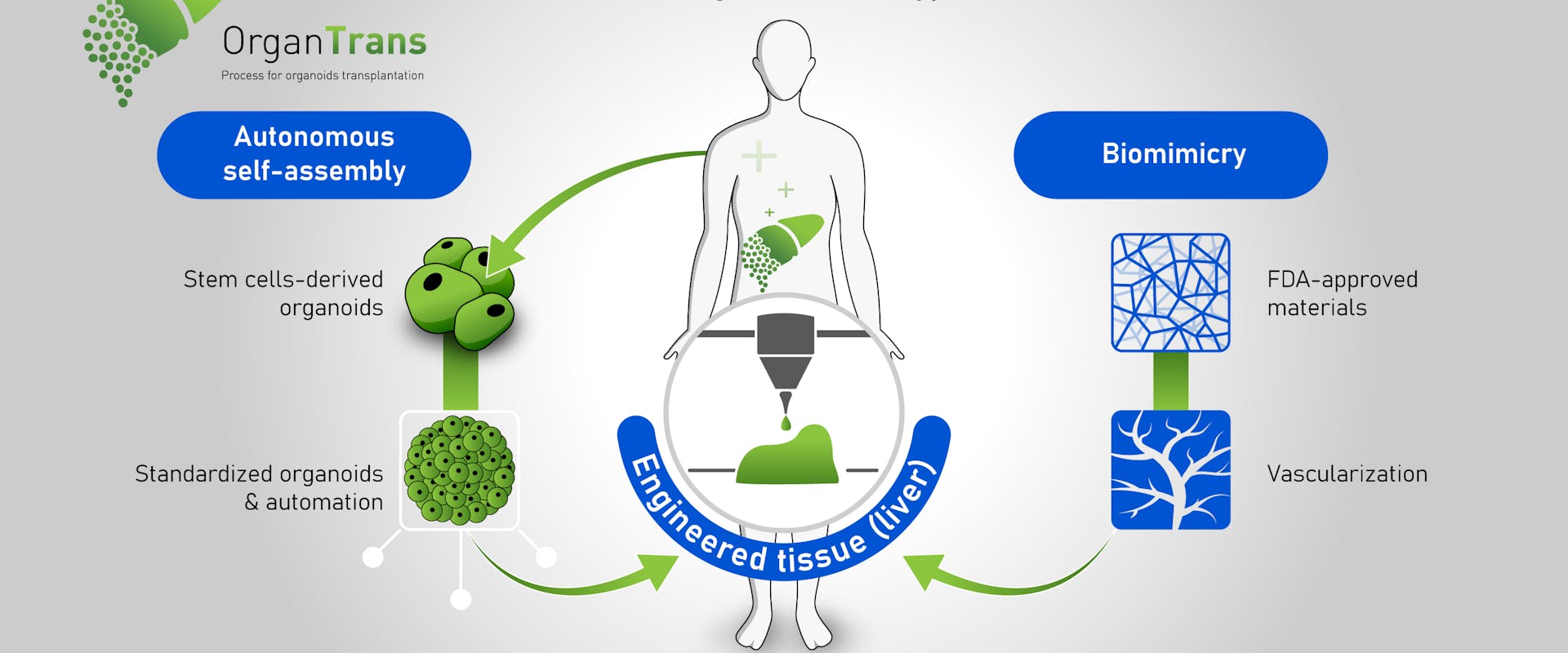
CSEM and ClexBio create a bioreactor for tissue-engineered cardiovascular implants
The teams at CSEM and at ClexBio have developed a process for engineering vein grafts comprised of human tissue material that integrates into the patient’s body and can turn into real, living tissue....

24 June 2021
CUTISS
CUTISS AG, a Swiss clinical-stage life sciences company focused on skin regenerative medicine and tissue engineering, has unveiled the world’s first automated machine to produce customized skin tissue...

3 February 2023
Making personalized cancer therapies accessible to all
CSEM has supported Lausanne-based start-up Limula in its mission to make cell therapies more affordable, and to facilitate access to these personalized treatments. ...